Veranova has a legacy of excellence in the separation and purification of drug substances. From impurity isolations to production-scale purifications, we have the expertise and equipment to support your needs.
Our full scope of instrumentation and techniques for chromatography, tangential flow filtration, and crystallization enable us to provide separations at every step along your drug development journey – whether you need to quickly purify your API to FDA standards, or identify impurities to improve processes for scale-up.
During this crucial stage of development, our separations team works closely with our analytical, chemical development and solid form experts. Leveraging this fully integrated approach, we purify an extensive range of APIs, including amorphous solids and lipids, drug polymer conjugates, ADC linker/warheads, and peptides and nucleoside analogs.
- Method development to identify modalities and optimum separation conditions
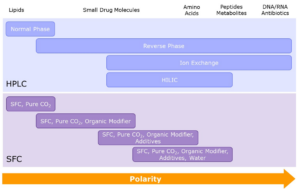
- Purifications – normal phase, reverse phase and supercritical fluid chromatography (SFC)
- Chiral separations of starting materials, catalysts, intermediates and APIs
- Impurity isolations, purifications and synthesis – nuclear magnetic resonance (NMR), mass spectroscopy (MS), and spectroscopies (Raman, IR and UV) are available for post purification analysis and identification