We have an in-house database of more than 150 chiral resolving agents to select from. This comprehensive list includes chiral acids, bases and coformers, complemented by information such as bulk price, solubility and pKa. As the number of commercial chiral acids with low pKa is limited, we have synthesised a set of strong chiral acids designed to form salts, such as chiral pyridines, that can be included in our chiral screens.
Using our know-how and experience of salt and cocrystal formation, a screen is designed with a subset of resolving agents selected with known synthons to interact with the target racemic molecule. For non-ionizable molecules, a chiral separation via the formation of chiral cocrystals could also be explored as part of a screening strategy.
Screens are typically performed using 20 to 30 mg of racemic mixture per experiment. The crystallization space is explored using multiple solvents (controlled cooling crystallization, liquid assisted grinding, anti-solvent additions, etc.). This approach maximizes the chance of identifying a suitable separating system for your chiral molecules. Our chiral screen service is supported by our solid state expertise in X-Ray Powder Diffraction (XRPD), Differential Scanning Calorimetry (DSC) and Thermal Gravimetric Analysis (TGA) and analytical team in chiral High Performance Liquid Chromatography (HPLC).
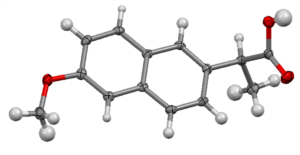
S-naproxen – thermal elliposids shown at 50% probability
Absolute stereochemistry can be confirmed by Single Crystal X-Ray Diffraction (SCXRD) internally by our experts and the information generated can complement the API dossier for FDA filling.
Robustness testing and scale-up of chiral separation are supported in-house. Commercial-scale chiral separation of API and intermediates is available at Veranova GMP and non-GMP facilities.
For molecules that are difficult to separate or purify by crystallization techniques, our Veranova team can also provide chiral chromatography methods for small and large-scale separations, including Supercritical Fluid Chromatography (SFC).


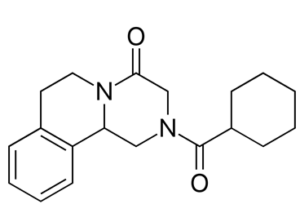 Praziquantel (PZQ) is an important racemic drug for the treatment of worm infections. It is a neutral molecule not bearing any functional groups which could allow a classical resolution by diastereomeric salt formation. The separation by cocrystal formation with L-malic acid was first reported in 2016 (Cryst. Growth Des. 2016, 16, 1, 307–314).
Praziquantel (PZQ) is an important racemic drug for the treatment of worm infections. It is a neutral molecule not bearing any functional groups which could allow a classical resolution by diastereomeric salt formation. The separation by cocrystal formation with L-malic acid was first reported in 2016 (Cryst. Growth Des. 2016, 16, 1, 307–314).