Screening and Method Development
Veranova has the latest in preparative chromatography technologies. We have fully automated screening by SFC and high-performance liquid chromatography (HPLC), enabling us to quickly assess the most effective instrumentation and modalities for your chromatography scale-up needs. At Veranova a diverse range of chromatography stationary phases, solvents and modifiers can be rapidly screened to enable quick development of optimal preparative separation conditions for all types of APIs. Our MS-coupled instrumentation enables our team of expert scientists to tackle purification of molecules without a chromophore.
Impurity Isolations
Our expertise in impurity isolations has proven valuable to our manufacturing team and clients alike. We can isolate and identify impurities from your intermediates and APIs removing any ambiguity. Our team of process chemists can then synthesize them for use as markers in your analytical investigations.
Post purification, our extensive analytical expertise allows us to accurately identify your unknown impurities. Veranova’s analytical instrumentation includes: NMR, MS, UV-Vis, FTIR, Raman, XRPD and polarimetry.
Controlled Substances
At Veranova, we have over 50 years experience in the development and manufacture of controlled substances and can provide chromatography support and expertise in this area. We perform purification of controlled substances at manufacturing scale and are licensed for Schedule I-V compounds. Veranova has an excellent relationship with the Drug Enforcement Administration (DEA).
Purifications
Veranova’s team of separation scientists are skilled at developing efficient purification methods for scale-up at development, kilo lab and manufacturing scale to achieve high purity APIs at every step of your process. We are able to provide GMP purifications across a full range of chromatography techniques at every scale.
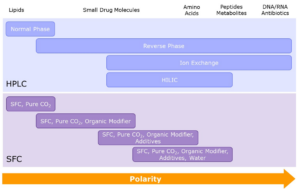
Diagram to show different purification techniques
Chiral Separations
Veranova can perform chiral separations for starting materials, catalysts, intermediates, and APIs. Veranova’s SFCs are ideal for manufacturing scale chiral separations. SFC is an excellent high–throughput technique for identifying optimal chiral separations methods. Using supercritical CO2 as a solvent allows SFCs to operate at much higher flow rates than HPLC giving quicker cycle times. The majority of SFC methods are isocratic which allows stacked injections which accelerate the purification times even further.
Purifications for Highly Potent APIs (HPAPIs)
Veranova has extensive expertise in HPAPI development, manufacture and purifications. Our toxicologists carry out rigorous safety assessments for all processes to ensure the correct controls are in place to safely handle potent compounds. Veranova has developed HPAPI purifications on six ADC programs and currently purifies HPAPIs at every scale from the development labs to manufacturing.

Veranova 1.2m chromatography column and processing infrastructure






