Read Expert Insight
Antibody-drug conjugates (ADCs): Navigating Four Pillars of Safety, Development, Supply Chain and Manufacturing Excellence
Our highly skilled process chemists and analytical scientists possess over a decade of experience developing and scaling up processes for linking small molecule payloads to polymers in support of antibody-drug conjugates (ADCs), polymer drug conjugates and other drug delivery applications.


Antibody-drug conjugates (ADCs): Navigating Four Pillars of Safety, Development, Supply Chain and Manufacturing Excellence
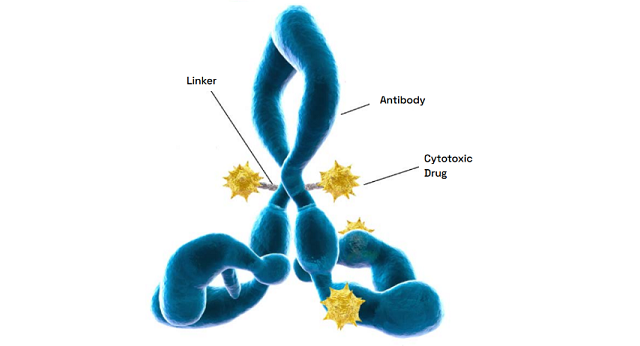
The ABC of ADCs: Fundamentals of Technical, Regulatory, and Clinical Insights

Antibody-drug conjugate development: challenges and opportunities

Discover new ways to advance your science with Veranova.
Contact us