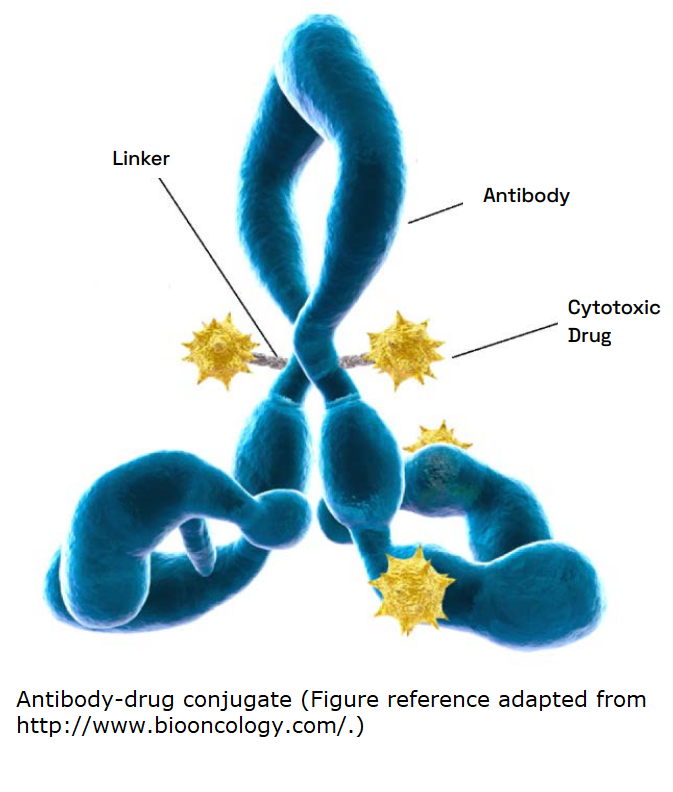
Over the past several decades, there has been a significant surge in the development of antibody-drug conjugates (ADCs). Designing an ideal ADC presents a multifaceted challenge, requiring the precise orchestration of various elements such as antigens, antibodies, linkers, and payloads. While ADCs aim to target tumor cells specifically, several antigens can also be found in regular tissues, potentially compromising the specificity of ADCs in therapeutic applications. The complexity extends to antibody selection, necessitating effective targeting of the desired antigen and ensuring compatibility with linkers for effective payload delivery. Additionally, the linker and payload combination are critical for the ADC’s therapeutic efficiency, balancing stability in circulation and timely payload release upon target binding. ADC doses must be safe for normal tissues while ensuring the released payloads are effective. The success of ADCs is attributed to their unmatched efficacy compared to traditional chemotherapy agents.
In this white paper, Veranova’s Global VP, AR&D, Dr. Kishore Hotha, provides a technical review of ADCs for cancer therapies, including a brief discussion of the basics of ADCs, regulatory approach, overview, and technical complexities for quantification.
This article summarizes recently approved ADCs and introduces the concepts of antibodies, linkers, and pay-loads. It also outlines cancer-specific ADCs currently in late-stage clinical trials for cancer treatment.