Understanding Chirality
Most of the organic chiral molecules in the pharmaceutical industry contain an asymmetric carbon center, meaning they have a unique three-dimensional shape and are not completely identical to their mirror image.
The human body is chirally selective, therefore it will interact with each component of a racemic drug differently and metabolize each enantiomer by a separate pathway. As such, the chirality of a molecule can influence key pharmacological activities, which impact a drug’s tolerability, safety, and bioavailability. As such, the control of enantiomeric purity and determination of individual enantiomeric drug molecules is a necessity, especially for clinical, analytical, and regulatory purposes.
Chirality in Drug Discovery & Development
In the early 1990s, the FDA recommended using optically pure drugs, requiring that the drug labeling should include a unique, established name and a chemical name with the appropriate stereochemical descriptors to specify strength, quality, and purity from a stereochemical viewpoint.
Furthermore, regulatory authorities in Europe, China, and Japan also provided guidelines indicating that only the active enantiomer of a chiral drug should be brought to market.
Since then, for pharma companies, single enantiomer drugs have become the standard when working with compounds containing asymmetric centers.
Shortening timelines for chiral drug discovery and development usually depends on the efficiency of enantiomeric separation and asymmetric synthesis. Therefore, it’s become increasingly crucial for developers to partner with organizations that can offer a reliable, high-quality chiral resolution to streamline drug development while still meeting project timelines and budgets.
Increasing Chiral Purity
Chiral resolution is often focused on a molecule’s solid form state. This is because the properties unique to enantiomers tend to manifest themselves predominantly in the crystalline state and it can be difficult to obtain single enantiopure compounds through solution-phase chemistry.
For today’s developers, there is a variety of tools available for separation techniques such as chiral chromatography, enantioselective membrane separation, and spontaneous or preferential crystallization. But as small molecule drug candidates in the development pipeline become more complex, choosing the right tool is increasingly important to avoid scale-up hurdles and minimize the barriers that could disrupt the journey to market.
Classical Resolution
Classical resolution via the formation of diastereomeric salt pairs and precipitation of the least soluble salts is one of the most widely used methods to produce optically pure enantiomers. This approach harnesses the variation in solid state and solubility properties between diastereomeric salts, by seeding racemic mixtures with pure enantiomer or a chiral resolving agent during crystallization. When successful, this is the best method for the manufacturer of a single enantiomer at moderate costs and is particularly amenable to scale-up.
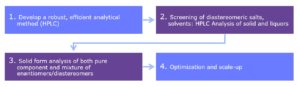
Figure 1: Diastereomeric Crystallization
Veranova’s Pharmorphix Solid Form and Particle Engineering team offer a classical resolution screening service to identify diastereomeric salts that are effective in the separation of racemic compounds into its two enantiomers. Initial screening experiments are performed in parallel, typically on a milligram scale to reduce material requirements and rapidly identify a suitable salt form.
The Pharmorphix team’s solid form experience is built on 2,000+ completed solid form projects across a range of structurally diverse and complex APIs. This provides us with a wide-ranging perspective of the development landscape, ensuring we’re able to quickly identify potential challenges and develop effective solutions.
In addition, to solubility differences, diastereomeric salts may exhibit different crystal morphology, thermal stability (melting point and melting enthalpy), processability, dissolution rate, hygroscopicity, and unequal tendency to crystallize. As such leveraging our history of excellence, Veranova is able to appreciate the need for thorough solid form characterization of the diastereomeric salts to provide a deeper understanding of the resolution process. This allows us to avoid the challenges caused by different polymorphic forms and/or solvates that can provide varying and conflicting resolution results.
Veranova’s capabilities for crystallization resolution
- Capability to develop custom chiral HPLC analytical methods
- Diverse selection of chiral acids and bases for resolution of racemic acids and bases, pre-selected for commercial availability
- Rapid screening and characterization of crystalline diastereomeric salts or cocrystals
- Comprehensive suite of complementary physicochemical techniques (e.g. DSC, TGA, XRPD, IR, RAMAN) for the characterization of physical properties of individual and combined crystalline forms
- Onsite single crystal X-ray diffraction (SXRD) service for structure determination and absolute stereochemical assignment including light atom only
- Optimization of crystallization procedure for scale-up using Process analytical technology (PAT) tools (in situ Raman or IR)
- Development of effective racemic synthetic processes
- Onsite kilo lab facilities for smooth process transfer
- Full-integrated capabilities for GMP manufacturing of clinical and commercial needs
An understanding of crystallization principles and techniques is an essential competence to develop an isolation process for enantiomerically pure material and underpins any commercial manufacturing processes.
Screening of chiral resolving agents
A non-rationalized approach to the screening of resolving agents can often result in poor recovery, low chiral purity, and inefficient, non-scalable crystallization protocols.
We aim to understand the crystallization process to optimize the separation conditions of the enantiomers.
- Development of suitable analytical methodologies such as chiral HPLC or GC if these are not available
- Screening of up to 70 chiral resolving agents which are available and appropriate for commercial-scale resolutions (with the option to screen in-house resolving agents)
- Screening performed in a broad range of solvents suited to forming diastereomeric salts
- Once crystalline solids are obtained, the diastereomeric excess (d.e.) is determined by chiral HPLC or GC.
- Solid state characterization of the crystalline solid.
- Determination of solubility points and assessment of efficiency of resolving agents
Resolution of neutral molecules
For small molecule compounds that lack an ionizable center, separation of racemic mixtures may be achieved by kinetic entrainment1 of conglomerates.
Crystallization of a conglomerate2 (racemic material composed of a 50:50 mixture of enantiomerically pure crystals) can give an efficient purification to provide the single enantiomer. For this, it is especially important to build up a full understanding of each particular system.
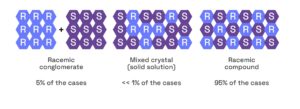
Figure 2: Crystal Forms of Racemic Mixtures
Using our industry-leading solid form understanding, Veranova can screen for conglomerates, having specific solid phase properties. Using phase diagrams constructed from thermal and solubility data, experiments are then designed to identify suitable entrainment through tightly controlled process parameters and specific crystal seeding methodology. A new technique known as Viedma ripening or attrition-enhanced deracemization has been developed in the last 10 years, this approach can also be employed to efficiently increase recovery of the desired enantiomer by utilizing grinding/milling technologies3, we have in-house wet milling capability to optimize such processes.
Cocrystal formation offers a new way of isolating chiral molecules; it is a variant of the classical chiral resolution but with chiral conformers. The molecule to resolve is forming enantiospecific interactions with a chiral former, allowing the precipitation of a solid cocrystal which is then isolated by filtration. The other enantiomer can be isolated from the mother liquor. The conformers are selected based on the potential intermolecular interactions they can form with the molecule to resolve. Single crystal data generated in-house can also help to design unique conformers which may be included in the cocrystal screen to maximize the chance of finding a suitable resolution system.
Recently, we have developed processes utilizing cocrystal technology to resolve racemic Praziquantel, the active ingredient of the antiparasitic drug, Biltricide. A simple process was developed after Dea Herrera-Ruiz4 in acetone to yield a cocrystal of L-malic acid/ Praziquantel in 98% de and 70% yield.
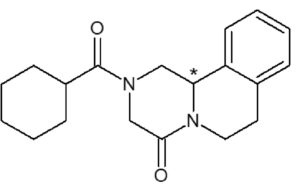
Figure 3: Structure of Praziquantel
Process Development and Kilo lab production
To develop a scalable process, crystallization development may be required to understand the process and kinetic activity, to avoid any problems upon scaling. Process analytical technology (PAT) and determination of points of the ternary phase diagram are employed to assess the process.
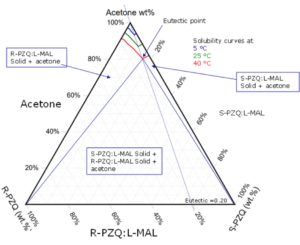
Figure 4: Phase diagram of L-malic acid/Praziquantel/acetone
Our onsite kilo lab can scale up and deliver material with full support from our crystallization team. In the last 15 years, we have developed chiral separation processes which have now been implemented for the production of commercial APIs.
Absolute Stereochemistry Determination
While developing an effective resolution or entrainment, determination of absolute stereochemistry can be achieved using single crystal X-ray diffraction. The state-of-the-art diffractometers at Pharmorphix allow structure elucidation with absolute stereochemical assignment of crystalline material, including light atom-only structures. These studies can be of critical importance in the support of regulatory submissions.
Supporting your success at every stage
Veranova has solutions to help you:
- Rapidly screen and identify single-enantiomer drug candidates for further testing.
- Support small-molecule drug discovery with stationary phases that isolate pure enantiomers effectively and efficiently.
- Protect your timeline by accelerating the selection of the optimal chiral column for your project.
- Improve analytical procedures with new methods that provide faster and more efficient analyses.
- Meet Pharmacopeia (USP/EP/JP/IP) and other industry quality standards for analytical methods.
1 P. Marchand, L. Lefebvre, G. Coquerel, Tetrahedron: Asymmetry 15 (2004) 2455-2665
2 J. Jacques, A. Collet, S. H. Wilen: Enantiomers, Racemates and Resolutions, Krieger Publishing Company Malabar, Florida, 1994
3 W. L. Noorduin, P. van der Asdonk, A. A. C. Bode, H. Meekes, W. J. P. van Enckevort, E. Vlieg,,B. Kaptein, W. van der Meijden, R. M. Kellogg, and G. Deroover, Organic Process Research & Development 2010, 14, 908–911
4 Jenniffer I. Arenas-García, Dea Herrera-Ruiz, Karina Mondragón-Vásquez, Hugo Morales-Rojas, and Herbert Höpfl, Cryst. Growth Des. 2016, 16, 1, 307–314





