Read our white paper
Analytical Techniques for Antibody-Drug Conjugates (ADCs): Comprehensive Insights
At Veranova, we bring over 19 years of experience in developing and manufacturing ADC linker-payloads to support biopharmaceuticals. Our comprehensive suite of services supports your journey from early development through to large-scale commercial manufacturing, ensuring you achieve optimal results at every stage. Our expertise spans payload-linker development and purification, all backed by our capabilities in highly potent active pharmaceutical ingredients (HPAPIs) and our world-leading cGMP facilities. Our site in Devens, MA is dedicated to delivering excellence in every aspect of linker-payload development.
“Working with Veranova has been a true partnership from day one. Their team was highly collaborative and responsive, making it easy for us to perform proof of concept work for our bioconjugation program. They quickly understood our needs, adapted with flexibility, and brought clear value to the table.”
– Pierre Launay, CSO, Inatherys
At Veranova, we offer a fully customizable approach to linker-payload development, scaling our processes to suit your project’s unique requirements. Our integrated services cover process development, analytical characterization, stability testing, and scalable production, ensuring seamless transitions from one phase to the next.
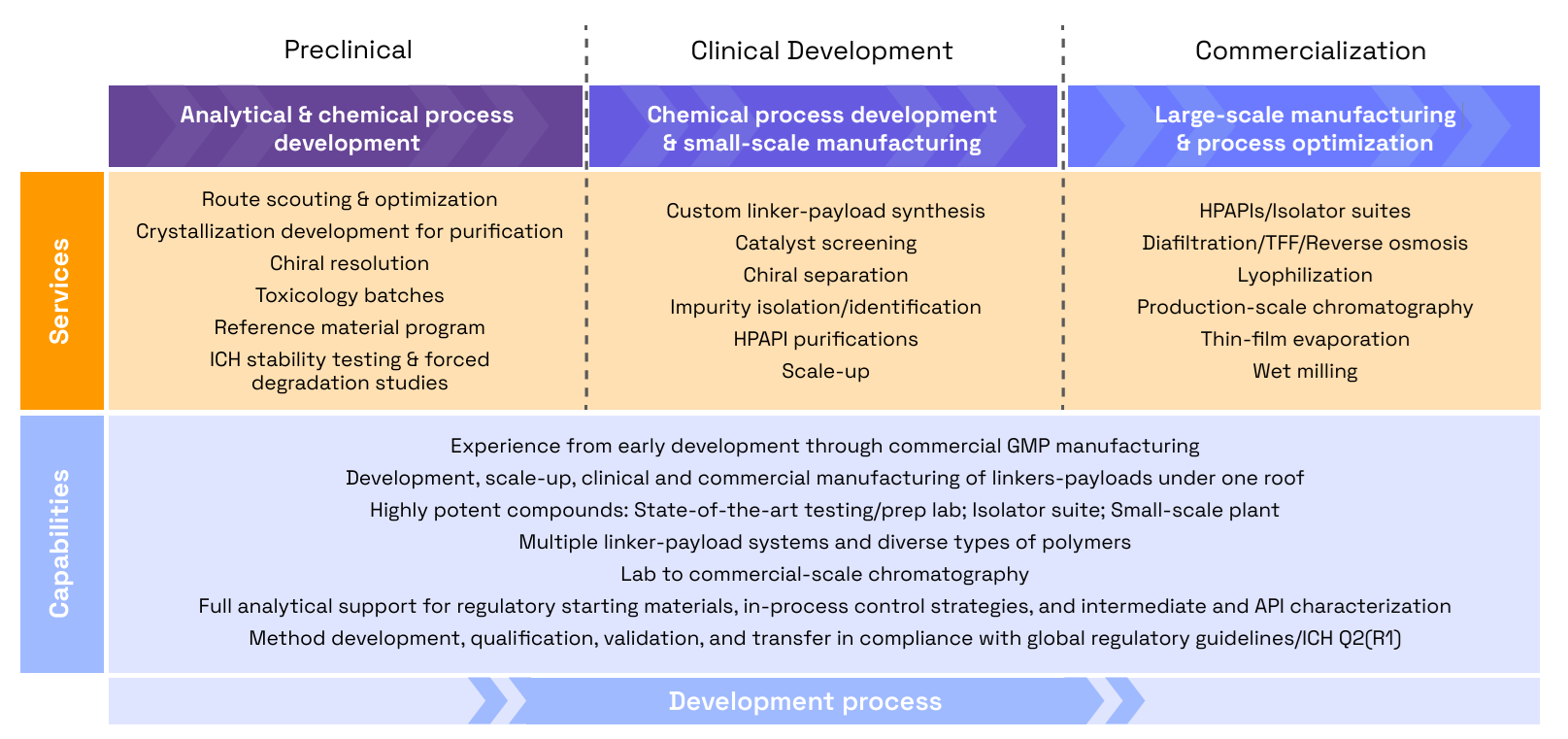
Veranova’s ADC services and capabilities
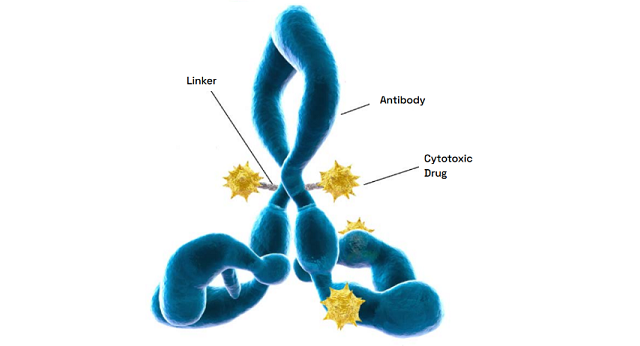
Expertise with multiple linker-payload constructs
Backed by years of proven expertise, we are ready to advance your molecule. Our deep knowledge of multiple linker-payload constructs allows us to tackle your unique ADC development and manufacturing challenges with confidence.
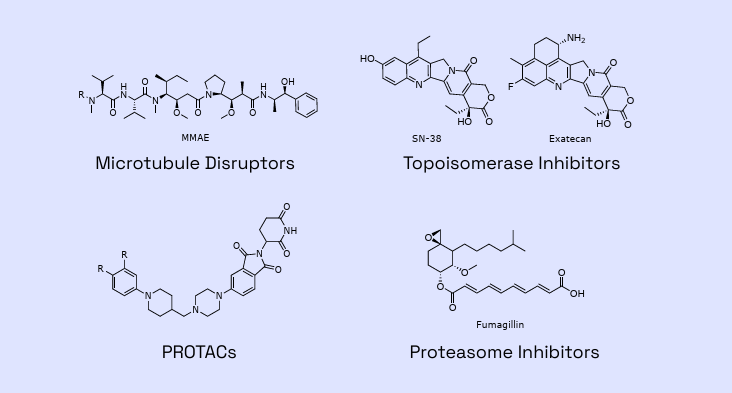
Figure 1: Examples of payloads within Veranova’s capabilities
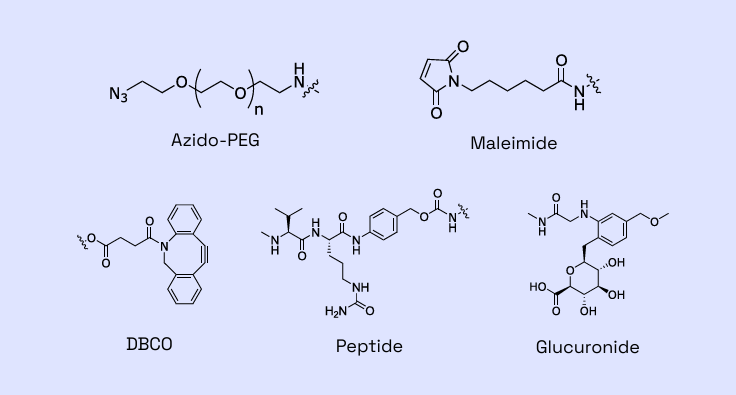
Figure 2: Examples of linkers within Veranova’s capabilities
Chromatography, purification & separations
We offer a full range of preparative chromatographic modalities at every scale. Leveraging cutting-edge knowledge and instrumentation, we can purify an extensive range of compounds in line with GMP standards.
Partnering for your success in ADC development and manufacturing
With Veranova, you’re choosing more than a service provider, you’re choosing a partner with a deep knowledge of complex chemistries and a proven track record in delivering innovative solutions. Supported by our dedication to quality chemistry, you can advance your ADC to its next phase with confidence.

Analytical Techniques for Antibody-Drug Conjugates (ADCs): Comprehensive Insights
Analytical Risk Management in ADC Development: From Lab to Plant

Understanding the Critical Role of Linkers in Advancing ADCs

Discover new ways to advance your science with Veranova.
Contact us