A proof-of-concept phase 2 trial recently demonstrated the significant and sustained efficacy of psilocybin, in combination with psychotherapy, for the treatment of alcohol use disorder (AUD).5 In another recent double-blind phase 2 study for TRD, a dose-dependent reduction in depression scores was observed in the weeks following administration of a single psilocybin dose.6 Similarly, a separate trial investigated the effects of two psilocybin-assisted therapy sessions for individuals with MDD. A significant reduction in depression scores was measured at the primary endpoint and at the four-week follow-up, and effects persisted for several months after administration.7 With noticeable effects after just one or two doses, the reduced administration frequency (compared to daily anti-depressants) improves treatment adherence leading to more successful patient outcomes.2
In addition, a growing number of phase 2 trials are currently underway to evaluate psilocybin’s potential across a broad spectrum of other conditions, including post-traumatic stress disorder (PTSD), OCD, bipolar II depression, anorexia nervosa, fibromyalgia, phantom limb pain, migraine, and distress associated with serious medical illnesses.2 It is also being investigated for its neuroplastic ability which has implications for neurodegenerative diseases like Alzheimer’s.8
Synthesis of Psilocybin
While psilocybin is naturally occurring in almost all mushrooms of the psilocybe genus (and in some other genera), isolating psilocin and psilocybin from mushrooms on gram scale is difficult. Additionally, the chemical content of the mushrooms can vary based on genetics, growing conditions, soil profile, and age. In clinical trials, both dose and purity must be precisely defined, as regulatory standards and scientific rigor demand strict control. In order to meet these demands, a robust synthesis is required. Recent clinical activities have gained momentum, and research interest in psilocybin is rapidly increasing, with several notable reports on its synthesis. Numerous synthetic methods for psilocybin have been reported to date,9 with the common approach involving the use of a benzyl-protected phosphorylating reagent, followed by deprotection. Kargbo and colleagues at the Usona Institute in Madison, Wisconsin, have worked extensively on the large-scale synthesis of psilocybin. They first developed a multi-gram scale, first-generation synthesis of psilocybin based on published methods.10 However, this initial approach encountered several challenges for scale-up. In response, they developed a second-generation synthesis, which involved the direct phosphorylation of psilocin on a kilogram scale.11
To demonstrate Veranova’s capabilities as a supplier of psychedelics, we performed a successful trial of the improved second-generation synthesis of psilocybin as reported by Kargbo et al.11 As shown in Scheme 1, psilocybin was synthesized in four steps starting from commercially available 4-acetoxyindole 1. Acylation of 4-acetoxyindole 1 in the presence of oxalyl chloride yielded compound 2. Treatment of 2 with dimethylamine provided ketoamide 3 as a solid. LAH reduction of intermediate 3 yielded psilocin 4. The direct phosphorylation of psilocin 4 was accomplished by treatment with POCl3 resulting in crude psilocybin. The crude psilocybin was further purified by re-slurrying in methanol and water, affording crystalline psilocybin in high purity (≥ 99.9%).
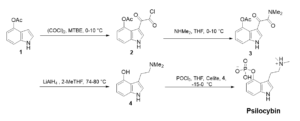
Scheme 1. Synthesis of Psilocybin.


 Cale Weatherly is Associate Director of Chemical Development at Veranova’s West Deptford, NJ site. He earned a PhD in Chemistry from the University of Wisconsin-Madison, developing synthetic organic methodologies under Prof. Jennifer M. Schomaker, and conducted postdoctoral research in natural products synthesis at the University of Pennsylvania under Prof. Amos B. Smith, III. Cale joined Veranova as a scientist in 2021, with prior work as a process chemist at Exemplify Biopharma and Abzena.
Cale Weatherly is Associate Director of Chemical Development at Veranova’s West Deptford, NJ site. He earned a PhD in Chemistry from the University of Wisconsin-Madison, developing synthetic organic methodologies under Prof. Jennifer M. Schomaker, and conducted postdoctoral research in natural products synthesis at the University of Pennsylvania under Prof. Amos B. Smith, III. Cale joined Veranova as a scientist in 2021, with prior work as a process chemist at Exemplify Biopharma and Abzena.