Pharmaceutical companies that partner with CDMOs offering cutting edge SCXRD services are increasingly leading the way in drug development. Through this partnership, the full potential of this powerful technique can be realized, providing companies with a competitive edge in today’s drug development landscape.
Understanding SCXRD
Within a crystal structure, atoms are arranged in an ordered repeating pattern, forming a crystal lattice that extends in all directions. SCXRD uses this, and the physical phenomenon of diffraction, to provide insight into the internal structure of the solid material. When X-ray radiation is directed at a single crystal, the X-rays interact with the planes of atoms in the crystal and diffract with different intensities and angles. The resulting X-ray diffraction pattern contains information about the 3D distribution of electron density within the crystal and can be used to determine the overall molecular geometry of the molecule.
Additionally, X-ray diffraction also provides insight into the nature of bonds between atoms and therefore the strength of intermolecular interactions between molecules in the solid state. SCXRD also has the benefit of being a non-destructive analytical method, meaning that the sample is not damaged in the process and analysis can be repeated if necessary.

Figure 1: Veranova’s SCXRD analytsis suite includes a fully automated and highly integrated Rigaku XtraLab Synergy-S diffractometer.
Driving development: invaluable insight into internal structure
Crystalline materials dominate the pharmaceutical landscape, with the vast majority of new and existing drug compounds being crystalline in nature. SCXRD, therefore, is an essential tool capable of confirming the chemical composition and molecular structure of a new chemical entity (NCE) from a single crystal. Crucially, SCXRD can assign the absolute stereochemistry of chiral centers, ensuring that the correct enantiomer is selected for development. having the desired activity at the target site. As well as streamlining the drug development process, this information is also vital from a regulatory standpoint, with stereochemical information being required for API regulatory and IP submissions of NCEs.
SCXRD is also important with regard to polymorphism. Crystalline APIs may exhibit polymorphism, which is when the same chemical compound exists in two or more different arrangements within the solid state. These polymorphs, or more broadly solid forms, when incorporating solvates (hydrate), salts and cocrystals have different 3D structures, including packing and bonding, giving rise to different physical properties, such as melting point and solubility. Additionally, any polymorphic form can impact the stability and overall biological activity of the API. Successfully identifying and producing any desired polymorphs during drug development is therefore essential. By providing insight into the different polymorphic forms of a drug, SCXRD along with other solid form techniques enables drug developers to gain a thorough understanding of the solid form landscape of the molecule in development. This also reduces the risk of processability and efficacy issues at a later stage of development.
At Veranova, we can rapidly screen samples to preliminary determine their crystal structure and suitability for full data collection. Our state-of-the-art Rigaku Oxford Diffraction XtaLAB Synergy-S diffractometer with dual X-ray source allows us to analyze a wide range of complex molecules, including peptides and other atypical moieties such as the glycopeptide Vancomycin (Figure 2).
Conducting variable-temperature SCXRD measurements can complement other thermal analytical techniques (eg. Differential Scanning Calorimetry (DSC). This can be used to monitor crystal-to-crystal phase transitions and understand the relationship between polymorphic forms, aiding in the selection of the most appropriate form for development.
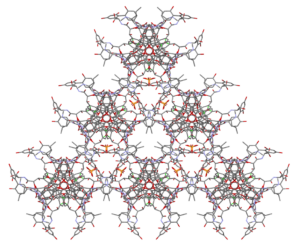
Figure 2: Complete structure of vancomycin phosphate
At the forefront of drug development
As areas of new development continue to emerge within the pharmaceutical industry, SCXRD has the potential to be used as a tool to guide and accelerate drug discovery and manufacture. One example of this is the formation of cocrystals, which are comprised of an API and a coformer, which may offer improved physicochemical and solid form properties. SCXRD can confirm the presence of both molecules in the unit cell, and in the instance where API:coformer are acid and base, that no proton transfer has occurred, making the material a cocrystal as opposed to a salt. This is important from a regulatory perspective, as both the FDA and European Medicines Agency require this. Moreover, understanding the solid form of an API, whether on its own or in the presence of other chemical moieties, early on, can alter the direction and scope of work, potentially saving time and money.
Investigating complex structures with confidence
Veranova’s cutting edge SCXRD facilities enable us to find impactful solutions to drug development challenges. In line with FDA guidance, we confirm a wide range of solid forms, including salts, cocrystals, and absolute configuration of chiral compounds. Using our powerful X-ray microfocus source, we can collect fast and accurate structure data on crystals historically considered as suitable only for synchrotron analysis.
Our in-house expertise and state-of-the-art equipment have a proven track record of excellence. In 2022, Veranova successfully determined over 100 crystal structures which made a real difference to progressing our clients API in development. Moreover, as part of our flexible and scalable offering, our services can be utilized for standalone analysis, but crucially also have the capability to be run in parallel with other solid form and particle engineering projects.
Under our Pharmorphix® brand, Veranova is trusted by its partners to provide innovative and effective solutions in early drug development. Our team of experienced crystallographers have over 20 years of invaluable SCXRD expertise, making us the ideal CDMO partner for your project. Utilizing our extensive analytical and characterization methods, we collaborate with our partners to maximize process efficiency and accelerate drugs to market.
For more information on our SCXRD offering, watch our video:




