Case Study: Liquid Phase Peptide Synthesis of Octreotide using Proprietary Tagging Technology
Octreotide is a cyclic octapeptide that serves as a more potent growth hormone inhibitor than its natural counterpart, somatostatin. This synthetic hormone is longer acting and more selective than the naturally occurring hormone. Octreotide is used to treat acromegaly, a disorder marked by the production of excess growth hormone, and it was proven to be over 20 times more active than somatostatin in initial in vivo models.iii Employing the LPPS approach, we have demonstrated the utility of our proprietary tag via liquid-phase synthesis of the commercial API, octreotide.
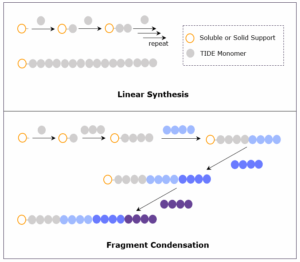
The liquid-phase synthesis of octreotide was performed utilizing a 6 + 2 fragment condensation approach, with the 6-mer fragment synthesized on tag (Scheme 1) and the dipeptide synthesized via general solution-phase. LPPS couplings and deprotections were carried out in dichloromethane (DCM) to ensure solubility of the tagged intermediates, but other solvents with lower environmental impact compatible with our technology have been identified.
2-mer Trt-tBu-Protected Octreotide Portion Synthesis: Fmoc-Cys(Trt)-OH was coupled with t-Bu-protected threoninol to form the 2-mer (H-Cys(Trt)-L-3(O-tBu)threoninol). After Fmoc-deprotection, the isolated solid was ready for coupling with the 6-mer portion described below.
6-mer N-Boc-Protected Octreotide Portion Synthesis (Scheme 1): After attaching threonine to the tag, the subsequent coupling reactions were performed with N,N-Diisopropylcarbodiimide (DIC)/Oxyma in DCM before the solvent was reduced and the product was precipitated by the addition of acetone as the antisolvent. Byproducts and unreacted starting materials were acetone-soluble, allowing for the isolation of the product by simple filtration. Fmoc-deprotection reactions were carried out in a piperidine/DCM system. After deprotection, the solvent was reduced, and the product was precipitated using acetonitrile as the antisolvent.
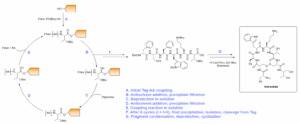
Scheme 1: Synthesis of Octreotide
Octreotide Fragment Condensation Synthesis (Scheme 1): Once the 6-mer was assembled on the tag, it was cleaved using basic conditions to preserve the acid-labile amino protecting groups. Cleavage from the tag furnished the N-Boc-protected 6-mer acid (Boc-D-Phe-Cys(Trt)-Phe-D-Trp(Boc)-Lys(Boc)-Thr(tBu)-OH), which was subsequently coupled with the 2-mer (H-Cys(Trt)-L-3(O-tBu)threoninol), followed by a global deprotection to produce linear octreotide. Cyclization to the ultimate target octreotide was performed using aqueous conditions under an atmosphere of open air.
Purification: Final purification of crude octreotide was performed using medium-pressure flash chromatography to afford octreotide as the acetate salt with a final purity of 92%. The purity was determined by liquid chromatography (LC, relative peak area with UV detection), and octreotide identification was confirmed by mass spectrometry (MS) of the main peak (Figure 2).
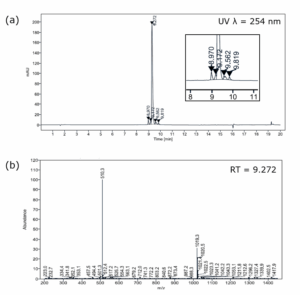
Figure 2. Chromatography of Octreotide Post-Purification. (a) Chromatogram for LC with UV Detection (Full-Scale Shown with Zoomed-in Inset) and (b) Mass Spectrum for Main Peak by LC-MS
We have successfully designed and synthesized an effective tag anchor for liquid-phase synthesis and demonstrated its utility with the synthesis of a cyclic commercial octapeptide, octreotide. Unlike peptide synthesis on solid support, liquid-phase synthesis of peptides on tags has no upper scale limitation, and we endeavor to achieve additional, customizable process development goals, including solvent optimization. We are confident in the advantages of liquid-phase reactions, including the use of lower solvent volumes and less expensive, more sustainable methodology for generating peptide APIs. We look forward to expanding our commercial and custom peptide portfolio using our proprietary tag compounds to deliver products with a more sustainable, efficient, and cost-effective approach.


 Alexander B. Koval, Ph.D., is a Senior Scientist in the Process Research & Development group at Veranova. He received his doctoral degree in chemistry from Temple University (Philadelphia, PA) in 2019, authoring papers on natural product analog synthesis, anti-cancer compound synthesis, and peptide synthesis. Alex started his industry career in 2019 and joined Veranova (formerly Johnson Matthey Health, West Deptford, NJ) in 2021. He is an inventor on two patents and has expertise in custom synthesis, process development, HPAPI synthesis, SPPS, and LPPS, among others.
Alexander B. Koval, Ph.D., is a Senior Scientist in the Process Research & Development group at Veranova. He received his doctoral degree in chemistry from Temple University (Philadelphia, PA) in 2019, authoring papers on natural product analog synthesis, anti-cancer compound synthesis, and peptide synthesis. Alex started his industry career in 2019 and joined Veranova (formerly Johnson Matthey Health, West Deptford, NJ) in 2021. He is an inventor on two patents and has expertise in custom synthesis, process development, HPAPI synthesis, SPPS, and LPPS, among others.