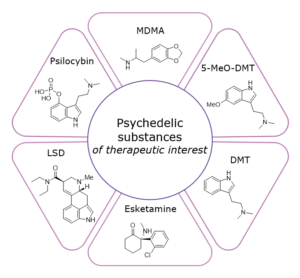
Figure 1: Psychedelic substances of therapeutic interest to the pharmaceutical industry
With a more holistic understanding of how psychedelics can be used in a supervised medical setting, scientists are beginning to explore their therapeutic applications again, especially in the treatment of illnesses such as addiction, depression, anxiety and posttraumatic stress disorder (PTSD)
However, there are several challenges associated with the quality, potency, safety, and efficacy of this resurgence in psychedelic substances. As a global CDMO that manages complexity with confidence, Veranova is positioned to help its customers mitigate these project hurdles and ensure consistent product attributes in the development and manufacturing of psychedelic substances for medical applications.
Psychedelic Substances of Interest | Potential Clinical Applications |
| Lysergic Acid Diethylamide (LSD) | Alcohol use disorder, anxiety disorders, depression, Alzheimer’s disease |
| Psilocybin | Several types of depression, smoking cessation, addictive behaviors, pain relief, migraine |
| 3,4-Methylenedioxmethamphetamine (MDMA) | Major depressive disorder (MDD), post-traumatic stress disorder (PTSD), anxiety disorders, eating disorders and other indications |
| Dimethyltryptamine (DMT) | Major depressive disorder (MDD) and other depression conditions |
| 5-MeO-Dimethyltryptamine (5-MeO-DMT) | Major depressive disorder (MDD) and other depression conditions |
| Esketamine | Treatment-resistant major depression, pediatric anesthesia, conscious sedation anesthesia, and emergency analgesia |
Table 1: Potential clinical applications for phychedelic substances
Complexity with confidence
Veranova leverages extensive experience in the handling of highly potent active pharmaceutical ingredients (HPAPIs) and controlled substances. We are committed to addressing the challenges that our customers face during the production, scale up, and process development of complex ingredients. Utilizing a wide range of services, Veranova can help develop high-quality, safe, and effective psychedelics.
We have expertise in analytical testing, chemical development, and manufacturing of controlled substances across our North American and UK sites. This includes the appropriate registrations to handle Schedule 1 substances. Furthermore, Veranova’s facilities and resources are well equipped to scale to GMP production of psychedelics for clinical use and meet increasing demands. Most recently, this has included an estimated $30 million expansion at our Devens, MA, site, as well as the completion of increased capabilities at our multi-purpose small and large-scale manufacturing site in Edinburgh, UK.
Regulation
Strict regulatory controls associated with controlled substances may pose a challenge to the continuing research and development of psychedelics. Most psychedelics are classified as Schedule I substances in the US and are similarly restricted in other countries. As a result, development and manufacturing activities require government licenses, inspections, and oversight. Veranova has a strong background in navigating regulatory frameworks to mitigate setbacks and efficiently maintain compliance and has extensive experience in the development and commercial manufacture of Schedule I drug substance.
Solid Form
In addition, Veranova can further develop the optimal solid form of a target psychedelic molecule through our world-leading solid form and particle engineering capabilities. Under our Pharmorphix® brand, Veranova can investigate the potential for the improved physical performance of an API under study via salt, cocrystal, and polymorphism screening protocols. We assist the selection of a solid form with desirable characteristics such as improved aqueous solubility, increased thermal stability and many other attributes. In conducting such work, we support IP generation and consequently help clients build a strong patent estate for their API. A typical workflow is given below (Figure 2).
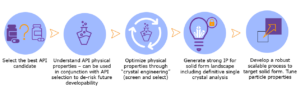
Figure 2. The steps of solid form drug development
Advancing Science with You
Veranova’s comprehensive range of services supports your drug development through strict regulation compliance and large-scale production. When conducted up front, solid form studies generate valuable IP and significantly reduce development time and costs. Our expertise and cutting-edge technologies can accelerate the development of psychedelics from drug candidate to commercial product.
Get in touch with our experts today to learn more about how we can accelerate your innovation with collaboration.
References
Tupper, K. W.; Wood, E.; Yensen, R.; Johnson, M. W. Psychedelic Medicine: A Re-Emerging Therapeutic Paradigm. Canadian Medical Association Journal 2015, 187 (14), 1054–1059. https://doi.org/10.1503/cmaj.141124.




