The therapeutic potential of psychedelic compounds has gained significant attention in recent years. While their use in conjunction with psychotherapy was briefly investigated during the 1950s and 1960s, such research was largely discontinued due to increasing regulatory constraints and societal stigma. Renewed scientific inquiry into these agents began in the late 1990s, with a gradual resurgence of preclinical and clinical studies thereafter.1 While pharmacologically distinct from classical psychedelics, 3,4-methylenedioxymethamphetamine (MDMA) is a psychoactive drug that behaves as an entactogen: it enhances empathy and emotional openness through its effects on serotonin, dopamine, and oxytocin systems.1,2 Clinical research has indicated that MDMA can help patients engage more openly in discussions about relationships, traumatic memories, and internal struggles, prompting mental health professionals to explore its integration into therapeutic practice.3,4 Since receiving regulatory approval for clinical research from the U.S. Food and Drug Administration (FDA) and Drug Enforcement Agency in 2004, MDMA has become one of the most studied psychedelics, second only to psilocybin.
Discover more about the synthesis of psilocybin for therapeutics.Synthesis of MDMA·HCl for Emerging Therapeutic Use

MDMA is one of the most promising therapeutic adjuncts to emerge in the pursuit of more effective and compassionate mental health care. MDMA-assisted therapy (MDMA-AT) produced encouraging results as a clinical approach for treatment resistant posttraumatic stress disorder (PTSD) in early pilot studies3,4,5 and phase 2 trials.6 This prompted the FDA to grant breakthrough therapy designation to MDMA-AT for PTSD in 2017.7 It has since been evaluated in randomized, placebo-controlled phase 3 clinical trials sponsored by the Multidisciplinary Association for Psychedelic Studies (MAPS). In the first phase 3 trial, 88% of completers with severe PTSD experienced a clinically significant reduction in symptom severity approximately 60 days after their third session of MDMA-AT, compared to 60% in the placebo group.8 In a confirmatory phase 3 study, 71.2% of participants in the MDMA-AT group no longer met the diagnostic criteria for PTSD at the primary endpoint versus 47.6% of participants in the placebo with therapy group.9 These results suggest that MDMA-assisted therapy is a promising and effective treatment for PTSD, offering substantial symptom relief with a strong safety profile under clinical conditions.8,9 Positive outcomes for PTSD treatment prompted pilot studies of MDMA-AT in autistic adults with social anxiety disorder and for psychological distress related to life-threatening illness.11 Both studies further highlighted the therapeutic potential of MDMA-AT. Participants in the Danforth et al. study had significantly reduced social anxiety at the primary endpoint and the six-month follow-up, and reported improved social interactions, confidence, and reduced barriers in relationships.10 Individuals with life-threatening illnesses who received three MDMA-AT sessions showed long-term reduction in anxiety and significant positive gains in mindfulness and social and family wellbeing.11
Following the success of clinical studies, Canada and Australia have introduced limited pathways for the legal prescription of MDMA-AT for PTSD. In 2022, Canada started allowing exception-based access via its Special Access Program when established treatment options were ineffective, not tolerated, or not accessible.12 In 2023, Australia’s Therapeutic Goods Association formally down-scheduled MDMA allowing for restricted prescription of this novel treatment for PTSD under a strict, medical framework.13 While these treatments show great promise, access is still highly limited with high cost and few providers.14 As the medical use of MDMA in regulated, clinical and therapeutic settings expands, there is a growing need for Current Good Manufacturing Practice (cGMP) synthesis methods to ensure product quality, safety, and regulatory compliance.
Demonstrations and Refinement of MDMA·HCl Synthesis
There are numerous successful synthetic routes to the preparation of MDMA·HCl described in the literature. Conventionally, most syntheses originate from either safrole or piperonal, both of which are classified as highly regulated precursors and are therefore difficult to procure.15,16 To improve accessibility and streamline preparation, Nair and colleagues at the MAPS Public Benefit Corporation (California), developed a fully validated, cGMP route that circumvents the use of these substances.1 Instead, they identified 5-bromo-1,3-benzodioxole 1 as a viable alternative starting material. Importantly, compound 1 is not listed as a controlled substance precursor by any regulatory authority. The 1,3-benzodioxole moiety is a structural motif commonly found in nature, present in essential oils, spices, and traditional plant-based medicines.
To demonstrate Veranova’s capabilities as a controlled substance manufacturer and trusted supplier of psychedelics , we conducted a lab-scale case study based on Nair et al.’s previously validated, multi-kilogram cGMP synthesis of MDMA·HCl.1 During development efforts, we implemented the established synthetic route using 5-bromo-1,3-benzodioxole 1 as the starting material. Slight modifications were made to address process-related challenges, enabling the successful production of multi-gram quantities of MDMA·HCl. As depicted in Scheme 1, the synthesis of MDMA·HCl was achieved through a three-step sequence starting from compound 1. In the first step, compound 1 (20.0 g) was transformed into the corresponding Grignard reagent, which was subsequently reacted with (±)-propylene oxide 2 to afford secondary alcohol 3 (15.4 g, 86%). Oxidation of alcohol 3 (12.0 g) using Dess–Martin periodinane produced the corresponding ketone 4 (10.1 g, 85%). The ketone 4 (10.0 g) was then subjected to reductive amination with methylamine and sodium borohydride, yielding the MDMA free base (9.8 g, 76.2%). The free base (9.5 g) was converted to the hydrochloride salt by treatment with HCl in isopropanol (IPA), affording crude MDMA·HCl (7.9 g, 70%) as an off-white solid. The crude MDMA·HCl (10.0 g) was recrystallized from IPA, furnishing crystalline MDMA·HCl (8.64 g, 86.4%) of high HPLC purity (≥ 99.9%).
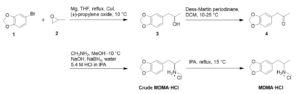
Scheme 1. Synthesis of MDMA·HCl
Challenges and Veranova Solutions
Key Considerations Identified During Development
The process chemistry team at Veranova identified several critical factors during route development that required close attention. These included careful control of physical conditions, strict management of reactant purity, and adherence to safety protocols.
a. The formation of the Grignard reagent in the initial step from bromo compound 1 is highly moisture sensitive, so it is essential to maintain strictly anhydrous conditions throughout the process. Upon successful formation, the Grignard reagent is first treated with copper iodide (CuI) and then reacted with propylene oxide 2. This is an exothermic reaction that requires slow addition to effectively control the heat generated, and efficient cooling to maintain safe reaction conditions.
b. Commercially available copper iodide (CuI) often contains impurities that can interfere with Grignard reactions, potentially leading to reaction failure. To ensure successful Grignard reaction, it is crucial to purify commercial CuI. This can be achieved through recrystallization from an aqueous saturated sodium iodide (NaI) solution.
c. Additionally, propylene oxide 2 is classified as a Veranova control band 4 hazardous material, necessitating stringent safety protocols during handling. Handling of the epoxide must be performed within an isolator, with personnel equipped with respirator masks and appropriate personal protective equipment (PPE).
Limitations of TEMPO Oxidation due to Impurity Formation
The procedure reported by Nair and co-workers used a (2,2,6,6-Tetramethylpiperidin-1-yl)oxyl or TEMPO based oxidation of compound 3 to form keto compound 4. This oxidation process led to the formation of side product via aromatic electrophilic chlorination of compound 4. These side products carried through to the subsequent steps and were detected in the final isolation of MDMA·HCl. To address this issue, the team at Veranova utilized an alternative oxidation method using Dess–Martin periodinane. The Dess–Martin oxidation proceeded cleanly, without the formation of side products, and yielded a high-purity keto compound 4 in good yield.
Laying the Foundation for Therapeutic Access
The growing acceptance of MDMA-AT in regulated clinical settings, along with limited international approvals, highlights the importance of establishing dependable synthetic routes to support safe, compliant large-scale production. To that aim, we successfully synthesized multi-gram quantities of MDMA·HCl starting from 5-bromo-1,3-benzodioxole 1, achieving both good yield and exceptional purity (≥ 99.9% by area). Remarkably, this high level of purity was obtained at the crude MDMA·HCl stage, eliminating the need for further recrystallization. The high crude purity was due in part from our team’s decision to address impurity formation during the oxidation step through use of Dess–Martin periodinane for the efficient conversion of the secondary alcohol to the corresponding ketone. This work underscores Veranova’s capabilities as a manufacturer of complex controlled substances. Building on these advancements, the Process Chemistry team at Veranova is well-equipped to address remaining challenges and deliver a cost-effective, robust, and scalable process for the multi-kilogram scale synthesis of MDMA·HCl to support safe and equitable access to emerging psychedelic therapies.
References
1 Nair, J. B.; Hakes, L.; Yazar-Klosinski, B.; Paisner, K. Fully Validated, Multi-Kilogram cGMP Synthesis of MDMA. ACS Omega 2022, 7, 900-907. https://doi.org/10.1021/acsomega.1c05520
2 Kargbo, R.B. MDMA and Their Analogs as Therapeutics for Mental Disorder and Response Predictor. ACS Med. Chem. Lett. 2022, 13, 1542-1544. https://doi.org/10.1021/acsmedchemlett.2c00389
3 Mithoefer, M.C.; Wagner, M.T.; Mithoefer, A.T.; Jerome, L.; Doblin, R.; The safety and efficacy of ±3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: the first randomized controlled pilot study. Journal of Psychopharmacology 2010, 25(4), 439-452. https://doi.org/10.1177/0269881110378371
4 Oehen, P.; Traber, R.; Widmer, V.; Schnyder, U. A randomized, controlled pilot study of MDMA (±3,4-Methylenedioxymethamphetamine)- assisted psychotherapy for treatment of resistant, chronic Post-Traumatic Stress Disorder (PTSD). Journal of Psychopharmacology 2012, 27(1), 40-52. https://doi.org/10.1177/0269881112464827
5 Mithoefer, M.C.; Wagner, M.T.; Mithoefer, A.T.; Jerome, L.; Martin, S.F.; Yazar-Klosinski, B.; Michel, Y.; Brewerton, T.D.; Doblin, R. Durability of improvement in post-traumatic stress disorder symptoms and absence of harmful effects or drug dependency after 3,4-methylenedioxymethamphetamine-assisted psychotherapy: a prospective long-term follow-up study. Journal of Psychopharmacology 2013, 27(1), 28-39. https://doi.org/10.1177/0269881112456611
6 Ot’alora, G.M.; Grigsby, J.; Poulter, B.; Van Derveer III, J.W.; Giron, S.G.; Jerome, L.; Feduccia, A.A.; Hamilton, S.; Yazar-Klosinski, B.; Emerson, A.; Mithoefer, M.C.; Doblin, R. 3,4-Methylenedioxymethamphetamine-assisted psychotherapy for treatment of chronic posttraumatic stress disorder: A randomized phase 2 controlled trial. Journal of Psychopharmacology 2018, 32(12), 1295-1307. https://doi.org/10.1177/0269881118806297
7 Multidisciplinary Association for Psychedelic Studies. (2017, August 30). MDMA-Assisted Therapy for PTSD Receives Breakthrough Therapy Designation by FDA. MAPS Newsletter: August 2017. Retrieved July 18, 2025, from https://maps.org/news/update/newsletter-august-2017/
8 Mitchell, J.M.; Bogenschutz, M.; Lilienstein, A.; Harrison, C.; Kleiman, S.; Parker-Guilbert, K.; Ot’alora, G.; Garas, W.; Paleos, C.; Gorman, I.; Nicholas, C.; Mithoefer, M.; Carlin, S.; Poulter, B.; Mithoefer, A.; Quevedo, S.; Wells, G.; Klaire, S.S.; van der Kolk, B.; …Doblin, R. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nature Medicine 2021, 27, 1025–1033. https://doi.org/10.1038/s41591-021-01336-3
9 Mitchell, J.M.; Ot’alora G.M.; van der Kolk, B.; Shannon, S.; Bogenschutz, M.; Gelfand, Y.; Paleos, C.; Nicholas, C. R.; Quevedo, S.; Balliett, B.; Hamilton, S.; Mithoefer, M.; Kleiman, S.; Parker-Guilbert, K.; Tzarfaty, K.; Harrison, C.; de Boer, A.; Doblin, R.; Yazar-Klosinski, B.; MAPP2 Study Collaborator Group. MDMA-assisted therapy for moderate to severe PTSD: a randomized, placebo-controlled phase 3 trial. Nature Medicine 2023, 29, 2473–2480. https://doi.org/10.1038/s41591-023-02565-4
10 Danforth, A.L.; Grob, C.S.; Struble, C.; Feduccia, A.A.; Walker, N.; Jerome, L.; Yazar-Klosinski, B.; Emerson, A. Reduction in social anxiety after MDMA-assisted psychotherapy with autistic adults: a randomized, double-blind, placebo-controlled pilot study. Psychopharmacology 2018, 235, 3137–3148. https://doi.org/10.1007/s00213-018-5010-9
11 Wolfson, P.E.; Andries, J.; Feduccia, A.A.; Jerome, L.; Wang, J.B.; Williams, E.; Carlin, S.C.; Sola, E.; Hamilton, S.; Yazar-Klosinski, B.; Emerson, A.; Mithoefer, M.C.; Doblin, R. MDMA-assisted psychotherapy for treatment of anxiety and other psychological distress related to life-threatening illnesses: a randomized pilot study. Scientific Reports 2020, 10, 20442. https://doi.org/10.1038/s41598-020-75706-1
12 Government of Canada. (2022, December 14). Access to drugs, including psychedelic drugs, through Health Canada’s Special Access Program. Question Period Note: December 2022 (MH‑2022‑QP‑0026). Retrieved July 28, 2025, from https://search.open.canada.ca/qpnotes/record/hc-sc%2CMH-2022-QP-0026
13 Therapeutic Goods Administration. (2023, February 3). Change to classification of psilocybin and MDMA to enable prescribing by authorised psychiatrists [Media release]. Department of Health and Aged Care. Retrieved July 28, 2025 from https://www.tga.gov.au/news/media-releases/change-classification-psilocybin-and-mdma-enable-prescribing-authorised-psychiatrists
14 Ducharme, J. (2023, February 8). The future of MDMA, psilocybin, and psychedelics in the U.S. Time. https://time.com/6253702/psychedelics-psilocybin-mdma-legalization/
15 Shulgin, A. T. The Background and Chemistry of MDMA. J. Psychoact. Drugs 1986, 18, 291−304. https://doi.org/10.1080/02791072.1986.10472361
16 Dunlap, L.E.; Andrews, A.M.; Olson, D.E. Dark Classics in Chemical Neuroscience: 3,4-Methylenedioxymethamphetamine. ACS Chem Neurosci. 2018, 9(10), 2408-2427. https://doi.org/10.1021/acschemneuro.8b00155
About the Authors
 Sanjeev Vernekar is an Associate Principal Scientist in Chemical Development at Veranova’s West Deptford, NJ site. He earned a Ph.D. in Organic Chemistry from the University of Muenster, Germany, where he focused on the synthesis of carba analogue of the natural product N-Acetylneuraminic acid under the guidance of Prof. Hartmut Redlich. Following his doctoral studies, he pursued postdoctoral research in Chemical Biology at the University of Warwick, UK. After moving to the United States, Sanjeev began his career as a Senior Researcher at the Center for Drug Design in Minnesota and subsequently worked as a Process Chemist at Thermo Fisher in South Carolina. He joined Veranova in 2021, bringing with him deep expertise in synthetic route development, process scale-up, and technology transfer.
Sanjeev Vernekar is an Associate Principal Scientist in Chemical Development at Veranova’s West Deptford, NJ site. He earned a Ph.D. in Organic Chemistry from the University of Muenster, Germany, where he focused on the synthesis of carba analogue of the natural product N-Acetylneuraminic acid under the guidance of Prof. Hartmut Redlich. Following his doctoral studies, he pursued postdoctoral research in Chemical Biology at the University of Warwick, UK. After moving to the United States, Sanjeev began his career as a Senior Researcher at the Center for Drug Design in Minnesota and subsequently worked as a Process Chemist at Thermo Fisher in South Carolina. He joined Veranova in 2021, bringing with him deep expertise in synthetic route development, process scale-up, and technology transfer.

Cale Weatherly is Associate Director of Chemical Development at Veranova’s West Deptford, NJ site. He earned a PhD in Chemistry from the University of Wisconsin-Madison, developing synthetic organic methodologies under Prof. Jennifer M. Schomaker, and conducted postdoctoral research in natural products synthesis at the University of Pennsylvania under Prof. Amos B. Smith, III. Cale joined Veranova as a scientist in 2021, with prior work as a process chemist at Exemplify Biopharma and Abzena.