A CDMO that Manages Complexity with Confidence
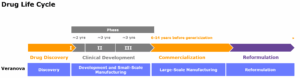
At Veranova, we specialize in the development, manufacturing and analytical testing of complex small molecules and novel modalities. As a leading drug substance CDMO, with over 50 years’ experience, our team of experts and cutting-edge instrumentation enables us to deliver life-changing complex chemistry solutions for our partners.
From ADCs (antibody-drug conjugates) to HPAPIs (highly potent APIs), we’re the partner with the depth and expertise you can trust to deliver.
Get in touch