Screening and Method Development Solutions
We specialize in a comprehensive suite of techniques for API separation, isolation, and processing. Our capabilities include crystallization, filtration, drying, lyophilization, as well as both wet and dry milling, and spray drying. We excel in spray drying APIs and amorphous solid dispersions (ASDs), encompassing a range of compounds such as small molecule APIs, lipids, antibody-drug conjugates (ADCs), peptides, and oligonucleotides.
Spray Drying Scales
Veranova has the capability to spray dry APIs from small-scale (200 mg) to multiple kilo-scale in our spray drying facilities in the UK and the USA.
Solvents – Aqueous or Organic
Water can be used to spray dry some APIs, but other APIs require the use of an organic solvent. However, organic solvents require special considerations due to their flammability, toxicity, and environmental impact. Veranova can handle organic solvents using an inert loop for spray drying. The solvent content in the inert loop is managed below the lower explosion limits (LEL) using built-in sensors and system interlocks.
Spray Analysis
HELOS Sympatec-based laser diffraction is used to characterize the nozzles for your API. We have developed a machine learning (ML) model to help with nozzle selection for your product.

Sympatec QICPC (left) and Sympatec HELOS RODOS (right) to analyze, monitor and measure particle size and shape
Product Characterization
Veranova utilizes a full suite of material characterization technologies, including the Phenom Scanning Electron Microscopy (SEM, Phenom Pharos) equipped with a Leica K3C camera, to analyze spray-dried materials in pharmaceutical products, particularly active pharmaceutical ingredients (APIs) and amorphous solid dispersions (ASDs). SEM provides high-resolution images that reveal the surface morphology and particle size distribution of spray-dried powders. This detailed visualization helps assess critical properties like particle shape, surface texture, and porosity, ensuring optimal solubility, stability, and bioavailability of the final product. In addition to SEM, Veranova employs a comprehensive array of analytical techniques for characterizing spray-dried materials:
- Particle Sizing (Malvern 3000)
- Polarized Light Microscopy (Leica, Nikon)
- Hot-stage Microscopy (Mettler Toledo)
- X-ray Powder Diffraction (XRPD) (Empyrean) with X-ray transparent tape-covered samples
- Thermogravimetric Analysis (TGA) using double-lidded pans
- Differential Scanning Calorimetry (DSC) using double-lidded pans
- Nuclear Magnetic Resonance (NMR)
- High-Performance Liquid Chromatography (HPLC) for purity and solubility testing
- Single Crystal X-ray Diffraction (SCXRD)
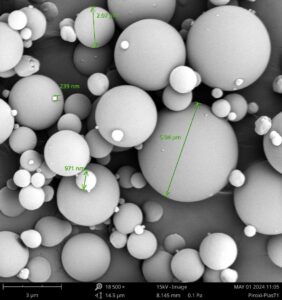
Piroxicam-Plasdone Amorphous Solid Dispersion Observed with Scanning Electron Microscopy
Spray Characterization
Veranova leverages expert know-how in spray analysis, for instance, with the advanced SprayScan mPT system, to capture detailed spray pattern characteristics, including size, shape, and distribution, enabling rapid verification of uniformity and coverage. By assessing the effects of different operating conditions, and documenting spray performance over time, the system facilitates process optimization and early detection of potential issues. This ensures Veranova’s spray-dried formulations achieve optimal solubility, stability, and bioavailability.
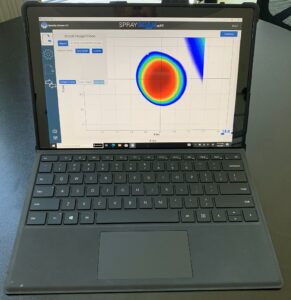
We use the SprayScan mPT that utilizes a laser and frame to illuminate the spray pattern for visualization
Controlled Substances
With 50 years of experience, Veranova specializes in supplying controlled substance APIs, including Schedule I-V compounds. We have extensive experience in manufacturing a wide range of controlled substances and opioids, ensuring reliable access to these regulated products and maintaining a secure supply. Our licenses for handling Schedule I-V compounds allow us to spray dry controlled substances at the kilo scale.
Highly Potent API Spray Drying
Veranova combines extensive technical expertise with specialized equipment to safely produce and handle highly potent APIs (HPAPIs). We utilize in-house isolators designed for effective containment practices that meet SafeBridge category 4 standards. Our isolator suites achieve an occupational exposure limit (OEL) as low as 0.01 μg/m³, and our small-scale manufacturing plants achieve a low OEL of 1 μg/m³. Our stringent adherence to low OEL levels ensures the safe and efficient production of HPAPIs, particularly for spray drying applications, leveraging our advanced technology and deep expertise.

Setup for HPAPI spray drying at Veranova






