Continuous Manufacturing (CM), or flow chemistry, is quickly becoming the future of drug substance manufacturing in the pharmaceutical industry, and CDMOs are leading the way in adopting this revolutionary technology. CM offers several advantages over traditional batch manufacturing, including increased efficiency, reduced waste, and improved product quality. By continuously processing materials, CM enables manufacturers to exercise more precise control over the manufacturing process, which results in fewer variations in the final product. This improved consistency can be essential for medicines that require exact dosing and uniform quality. Furthermore, using CM allows for more streamlined and flexible manufacturing processes, which is particularly beneficial in the face of growing demand for personalized medicine and smaller batch sizes. As such, CDMOs are making significant investments in new facilities and equipment to support CM, driving innovation in the industry. With the potential to reduce lead times, cut costs, and improve product quality, it is clear that CM will play a significant role in the future of drug substance manufacturing.
Continuous manufacturing (flow chemistry) – the future of pharmaceutical manufacturing

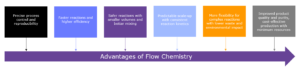
Drivers for the implementation of flow chemistry processes
Process efficiency
The implementation of flow chemistry processes in drug substance contract development and manufacturing organizations (CDMOs) can be driven by several factors, including:
- Improved efficiency and productivity: Flow chemistry processes can significantly improve efficiency and productivity compared to traditional batch processes. The continuous flow of reagents and products can lead to faster reaction times, better yields, and reduced waste, resulting in a more efficient and streamlined manufacturing process1.
- Greater flexibility and scalability: Flow chemistry processes can be easily scaled up or down to meet changing production demands, making them highly flexible and adaptable. This can be especially important for CDMOs that work with a variety of clients and need to be able to adjust quickly to different project requirements.
- Enhanced safety and environmental sustainability: Flow chemistry processes can be designed to minimize hazardous reagents and solvents, resulting in a safer and more environmentally sustainable manufacturing process. This can help CDMOs meet increasingly stringent regulatory requirements and reduce their environmental impact.
- Competitive advantage: Implementing flow chemistry processes can help CDMOs differentiate themselves from their competitors and attract new clients seeking more efficient, flexible, and sustainable manufacturing solutions.
Innovation
Continuous manufacturing has the potential to drive innovation in several ways and can be particularly beneficial for biotech companies. Unlike traditional batch manufacturing, continuous manufacturing allows for a more streamlined and efficient process that can lead to cost savings, higher productivity, and better control over the final product.
- Increased process control and understanding: Continuous manufacturing allows for real-time monitoring and control of manufacturing processes, which can lead to a greater understanding of the underlying chemistry and the ability to optimize process conditions. This can result in more efficient and reliable manufacturing processes and the development of new and improved products.
- Faster process development and scale-up: Continuous manufacturing can significantly reduce the time and resources required for process development and scale-up compared to traditional batch processes. This can enable manufacturers to bring products to market more quickly and efficiently and facilitate the development of new and more complex products.
- Integration of new technologies: Continuous manufacturing often involves advanced technologies such as process analytical technology (PAT), which can provide real-time monitoring and control of critical process parameters. This can enable manufacturers to identify and address issues more quickly and facilitate the integration of new technologies into the manufacturing process.
- Increased agility and responsiveness: Continuous manufacturing allows for greater flexibility and agility in responding to demand or product requirements changes. This can enable manufacturers to rapidly adjust production volumes and product specifications and facilitate the development of new and customized products.
Supply chain advantages
Supply chain security and equipment advantages are also strong emerging drivers for the adoption of CM.
- Improved process control and quality assurance: Continuous manufacturing allows for real-time monitoring and control of critical process parameters, which can help to ensure consistent product quality and reduce the risk of product defects or deviations. This can help to minimize the risk of supply chain disruptions and provide a reliable supply of high-quality products.
- Reduced lead times and inventory requirements: Continuous manufacturing can significantly reduce the time and inventory required for manufacturing compared to traditional batch processes. This can help reduce stockout risk, minimize inventory costs, and improve overall supply chain efficiency.
- Greater flexibility and responsiveness: Continuous manufacturing enables CDMOs to rapidly adjust production volumes and product specifications in response to changing customer demand or regulatory requirements. This can help to improve overall supply chain agility and responsiveness.
- Enhanced sustainability: Continuous manufacturing can help to reduce waste, energy consumption, and carbon emissions, which can improve the sustainability of the supply chain. This can be an essential factor for customers and stakeholders increasingly focused on environmental sustainability.
Understanding & overcoming implementation barriers
To overcome these challenges, global health authorities and pharmaceutical companies must work to increase awareness of the benefits of CM throughout the industry. Developing a regulatory framework for CM would also help promote its widespread adoption. At the same time, more significant investment from manufacturers into employee training would equip staff with the knowledge to implement flow chemistry techniques.
To access the expertise and equipment, pharmaceutical companies are increasingly turning to CDMOs. Partnering with an organization with flow chemistry capabilities enables manufacturers to overcome development barriers, resulting in a streamlined process.
Implementing continuous manufacturing in drug substance contract development and manufacturing organizations (CDMOs) can face several barriers that can be categorized as technical, operational, and cultural. Some of the most common implementation barriers for continuous manufacturing in CDMOs include:
- Technical challenges: Continuous manufacturing can involve complex equipment and process design requiring specialized knowledge and expertise. Implementing continuous manufacturing may require significant capital investment and a steep learning curve for operators and other personnel.
- Operational challenges: Continuous manufacturing can require significant changes to existing manufacturing processes and workflows, which can be challenging to manage and may require new or modified standard operating procedures (SOPs). This can also involve a shift in mindset from a batch-based to a continuous-based approach.
- Cultural challenges: Implementing continuous manufacturing can require a shift in organizational culture, particularly around risk management, quality assurance, and decision-making. This can require significant buy-in and support from senior management and other stakeholders.
To overcome these barriers, CDMOs may need to take a structured and collaborative approach to implementation, focusing on the following strategies:
- Invest in training and education: Continuous manufacturing requires specialized knowledge and expertise. Investing in training and education can help ensure that personnel has the necessary skills and expertise to implement and operate continuous manufacturing processes effectively.
- Develop new standard operating procedures (SOPs): Implementing continuous manufacturing may require new or modified SOPs to accommodate the different process and workflow requirements. Developing clear and concise SOPs can help to ensure that all personnel are following the same procedures and best practices.
- Engage stakeholders: Engaging stakeholders, including senior management, operators, and customers, can help to ensure that everyone is aligned around the benefits and requirements of continuous manufacturing, and can help to build support for implementation.
- Pilot test and iterate: Piloting and testing continuous manufacturing processes on a smaller scale can help to identify and address technical, operational, and cultural challenges before scaling up. Iterating on the pilot results can help to refine the process and ensure that it is optimized for efficiency, quality, and reliability.
Managing flow chemistry with confidence
At Veranova, our unwavering commitment lies in handling intricate and state-of-the-art drug development with the utmost confidence. As part of our custom pharma solutions portfolio, we offer fast and phase-appropriate process development, which includes utilizing flow chemistry for the laboratory to commercial-scale production. By capitalizing on the modular and adaptable nature of the continuous flow, we are empowered to discover ground-breaking solutions for synthesizing premium pharmaceutical ingredients.
Combining the attributes of the ideal CDMO partner
With over five decades of experience in the pharmaceutical industry, Veranova prides itself on providing a bespoke approach to every molecule, handpicking the most appropriate techniques to swiftly ascertain the most appropriate formulation technology. Drawing on our broad array of analytical and characterization methodologies, we work with our clients to optimize process efficacy and attain their drug product’s target product profile.
About the author
Dr. Kishore Hotha is the scientific leader at Veranova and serves as the Global Head of Analytical R&D. With a career spanning over 20 years: he has provided scientific business leadership for numerous INDs, NDAs, and ANDAs. Dr Hotha has extensive expertise in developing drug substances and products, particularly in HPAPI, Drug linkers, ADCs, and complex drug dosage forms. He has authored over 40 scientific research publications and serves on several editorial boards. Dr. Hotha received his Ph.D. from JNT University, India, and has held leadership positions at Lupin and Dr. Reddy’s in Research & Development.