In 2023, Veranova carried out work for a client designing a robust crystallization process to provide access to one of a pair of anhydrous polymorphs. Wet milling was integrated into the process to yield particles of the target solid form with desired particle size attributes.
Problem
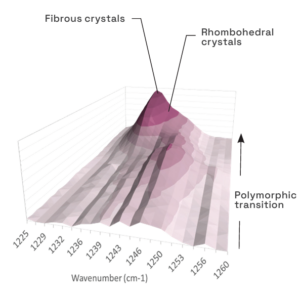
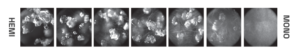
The legacy process at the time resulted in the isolation of physical mixture of two anhydrous polymorphs. The relative stability of these forms was unknown and thus the desired form for further development was not defined. Due to formulation and downstream processing requirements, a narrow particle size distribution, with Dv50 of ca. 20 µm, was also requested from the process design.
Scope of work carried out at Veranova
- Competitive slurry experiments successfully identified the more stable of the two anhydrous forms which was selected as the form for further development.
- Solubility measurements and process modelling enabled design of a combined cooling/anti-solvent addition process.
- Good solid-form control was established using this procedure with seeding at multi-gram scale with a firm understanding of the process gained via PAT tools (Blaze Probe).
- Control of the solid form and design of a growth dominated process led to larger crystals than requested by the client so milling strategies were explored.
- A terminal wet milling step, using an IKA® magic LAB® with MKO headset, was introduced into the process which allowed a 4-fold reduction in Dv50 to within the limits requested by the client. The milling process was optimized by tracking particle breakage in-line with PAT.
- The final demonstration batch was carried out successfully in a 10 L vessel, at 0.5 Kg scale.
Outcome
Client was able to select the most stable form of the drug substance to take forward. A robust crystallization process was designed and demonstrated at 0.5 Kg scale including a terminal milling step to fine-tune the particle size distribution to the value requested by the client.

 Pictorial representation of different crystallization pathways with respect to solubility and MSZW
Pictorial representation of different crystallization pathways with respect to solubility and MSZW