By understanding the intrinsic properties of the drug substance, we are able to develop prototype formulations that improve the biological performance of a drug candidate and maximise the chances of success in preclinical studies.
Through our in-house solid form analytical and preformulation team, we are able to support analytical requirements for IND applications and support early phase clinical studies. As a non-GMP facility we are able to work with maximum efficiency and flexibility to meet customer milestones.
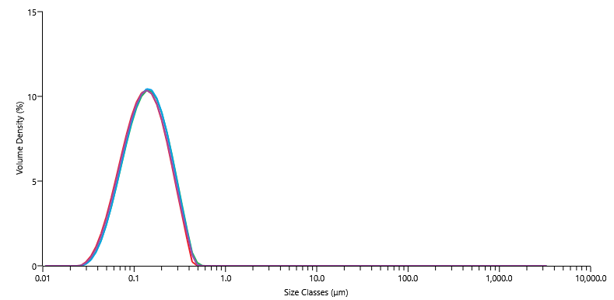
Scouting studies for enabling formulation encompasses a wide range of screening methodologies and analytical techniques, depending on the intended administration route.
Download our Enabling Formulation Flyer