Nucleotides and Oligonucleotides
Custom synthesis with modifications to backbone linkages, sugars, and bases.
Veranova tackles your unique TIDES development and manufacturing challenges to deliver cost-effective, high-quality, greener, scalable solutions. We are an experienced CDMO partner in TIDES pipelines with over 10 years of experience in the development to commercial-scale production of nucleotides and drug modifiers, and in the development of peptides and oligonucleotides.
We offer custom synthesis, multi-scale production, purification, and finishing, supported by a dedicated team of experts. Our world-class capabilities include:

Custom synthesis with modifications to backbone linkages, sugars, and bases.
Custom synthesis including natural and unnatural amino acids.
Custom Nucleotides, Oligonucleotides, Peptide, and Drug Modifier Production
Veranova has extensive, expert knowledge in process chemistry, process engineering, and analytical testing to support the customized production of your drug target. We can produce modified nucleotides and unnatural amino acids for custom TIDES and utilize solid-phase, liquid-phase, and/or fragment condensation synthesis for TIDES active pharmaceutical ingredients (APIs) production—offering flexible end-to-end supply chain management.
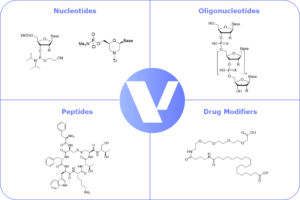
Figure 1: Veranova products for TIDES pharmaceuticals
Complete Support and Cost-Effective TIDES Solutions
Veranova’s expertise in the production of complex molecules ensures an efficient program and a high-quality product. We provide modern and unique manufacturing techniques and services that can quickly advance your drug from development to commercial production. Our technical and project teams provide comprehensive support for TIDES drug programs by leveraging our extensive capabilities and knowledge in TIDES production, process optimization, program development, and regulatory life cycle.
Technical Capabilities in TIDES Drug Program Support
Our synthesis, isolation, purification, and analytical capacity provides the technical foundation for a successful partnership. We have strong experience in:
Our decades of experience assure high-quality products and a secure supply chain in the production of TIDES, nucleoside phosphoramidites, and diverse drug linkers and modifiers including polyethylene glycol moieties (PEGs), lipids, and other small molecules. Our purification capabilities include preparative chromatography at all scales, with a variety of dynamic axial compression columns up to 1.2 m in diameter. Our distinctive solid form science team can also assist with optimization of process efficiency, materials’ stability, and/or final drug substance pharmacokinetics. Successful innovation outcomes and high-quality products are ensured by our analytical method development and testing teams who are equipped with state-of-the art techniques and extensive technical background. Together, our technical teams produce custom molecules for TIDES drug programs with optimized efficiency and molecular characteristics.
Innovative Manufacturing Techniques
Veranova provides added value technology by offering a variety of options in synthetic and isolation approaches. Both liquid and solid phase synthesis may be developed as well as leveraging proprietary methodologies and fragment condensation for improved efficiency and quality. Solvent, starting materials, and production time may be reduced to realize environmental and economical cost benefits. Veranova’s proprietary procedures also enable improved quality through increased in-process control testing junctures, improved reaction kinetics, and higher purity. Additionally, manufacture of linkers, sidechains, and conjugate supports may utilize our flow chemistry capabilities (up to 24 kg/day) and other innovative techniques. We also offer pioneering solid form and particle engineering practices that include spray drying and crystallization/precipitation options for isolation.

Discover new ways to advance your science with Veranova.
Contact us