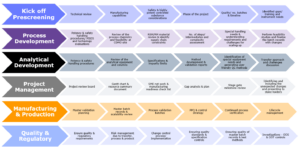
Veranova can support you through the entire scale-up and technology transfer process.
We work closely with customers to ensure that knowledge and technologies are transferred seamlessly between the phases of drug development, allowing for a more efficient and cost-effective drug development process.
We offer a fully integrated approach; our experts in process and analytical chemistry, quality and production work together to produce solutions for scale-up, either at one of our GMP manufacturing sites or to another facility.
- Expert project management services
- Multidisciplinary team work together to support knowledge transfer
- Method development and validation
- GMP manufacturing sites across the UK and US enable efficient tech transfer from development to commercial production
- Highly potent API handling expertise
- Quality and regulatory team provide governance and guidance throughout the process