Watch Webinar:
Application of Solid Form and Crystallization Science in Purification Strategies of New Chemical Modalities (NCMs) including Peptides and PROTACs.
PROTACs present unique challenges in drug development and manufacturing due to their distinctive molecular properties and potency.
Veranova is experienced in handling highly potent active pharmaceutical ingredients (HPAPIs) and has extensive capabilities in separation technologies, solid form sciences, and crystallization development to successfully address the many challenges accompanying chemical development and manufacturing of PROTAC molecules.
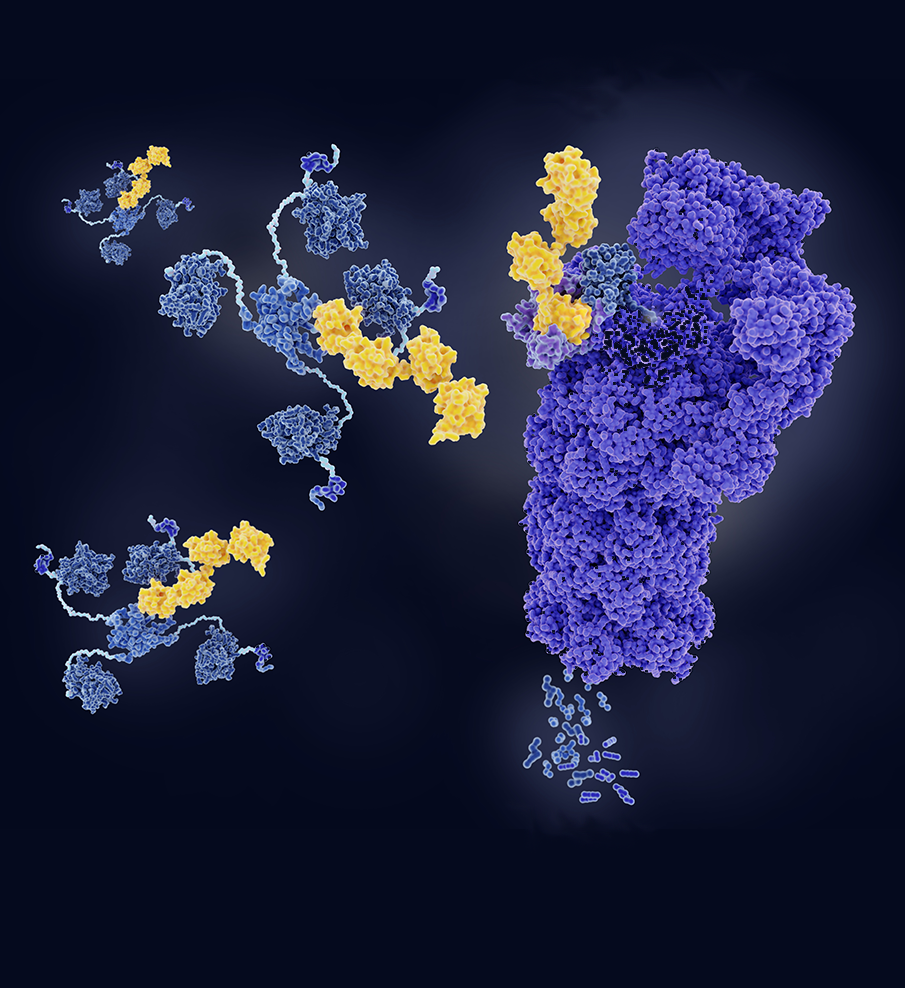
PROTACs are heterobifunctional molecules that target hard-to-drug proteins to treat diseases like cancer, diabetes, and Alzheimer’s. They consist of a targeting ligand for a disease-causing protein paired with an E3 ubiquitin ligase ligand through a flexible linker.
PROTACs typically violate one or more of Lipinski’s Rule-of-Five (Ro5) due to their high molecular weight (> 500 Da), excessive H-bond donors/acceptors (> 5/10) and elevated lipophilicity (cLogP > 5). These properties compromise their pharmacokinetic profile, particularly oral bioavailability and aqueous solubility. In addition, these compounds are typically highly potent and require specialized handling in R&D and production. PROTACs may also be challenging to isolate as crystalline solids (desirable at scale), owing to their incorporated flexible linkers.
HPAPIs and Complex Chemistry
PROTACs require facilities designed to handle HPAPIs. In addition, the inherent flexibility in the molecules due to the nature of the linker often presents challenges in purification. Chromatography is a powerful tool, offering critical purification capabilities that complement and enhance crystallization efforts.
Improving PROTAC’s Bioavailability
The bioavailability of poorly water-soluble drugs is a significant challenge in drug development, affecting many new chemical entities. For oral dosage pharmaceutical forms, aqueous solubility is a crucial, fundamental factor for achieving bioavailability and therapeutic activity.
Our specialized, highly experienced team is adept at overcoming challenges related to the solubility and bioavailability of APIs which are common to PROTACs:
Optimizing PROTAC’s Crystallization
With our extensive in-house experience in crystallizing complex molecules, including PROTACs, Veranova is able to confidently tackle their common inherent challenges. We implement well-designed crystallization workflows for molecules with flexible linkers that hinder crystallization. We leverage salt and cocrystal formation strategies to investigate crystallinity often in parallel with solid form investigations to optimize aqueous solubility. We design robust crystallization protocols to enhance the developability (isolation, stability, and scalability) of lead candidates and intermediates.
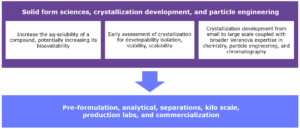
Figure 1. Flowchart illustrating the development and scale-up of PROTACs.
Veranova provides a wide range of services for full lifecycle of drug development to commercial manufacturing. We can offer one or multiple services simultaneously to meet your specific requirements.
Application of Solid Form and Crystallization Science in Purification Strategies of New Chemical Modalities (NCMs) including Peptides and PROTACs.

Crystallization Process Development

Discover new ways to advance your science with Veranova.
Contact us