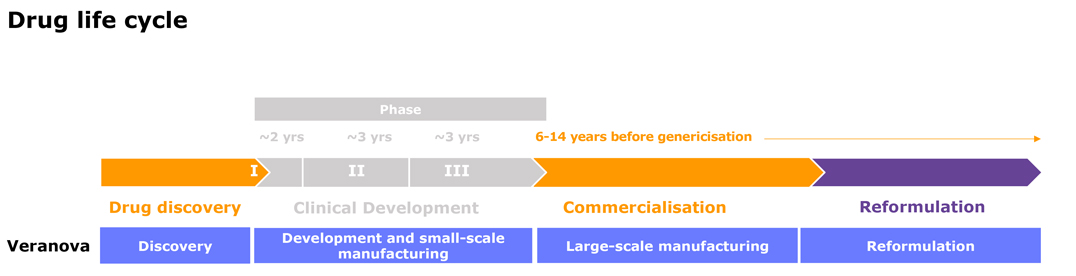
Process Development
Our dedicated process development chemists can help you optimize your drug development and manufacturing processes.
At Veranova, we have over 50 years’ experience navigating the challenges of the global pharmaceutical industry. Through our custom pharma CDMO services, we can help you manage your most complex chemistry and scale-up challenges with confidence.
We offer complete custom services, from pre-clinical to commercialization, providing vital pharmaceutical development, production and analysis solutions at our global network of dedicated manufacturing sites. Our team of experts and cutting-edge instrumentation enables us to deliver life-changing complex chemistry solutions for our customers.
Learn More About Our Custom Pharma Solutions
We are committed to building strong relationships with our customers. By developing a true understanding of your needs and aims, we provide the right solutions to accelerate your product to market.
Our team of skilled scientists have extensive expertise in the fastest growing areas of development in the pharmaceutical industry today, and can anticipate the key challenges you may encounter throughout the drug development process. Keeping your project on track is our priority.

At Veranova, we are committed to fostering excellence through collaboration. Trusted for our knowledge and experience in pharmaceutical product development and manufacturing, we work closely with our customers to overcome challenges during drug development.
Utilizing efficient technology and knowledge transfer, Veranova is able to improve process efficiency, meet clinical material and timeline needs, and scale processes up to commercial level when needed.
As a CDMO partner, we leverage our expert knowledge of complex multi-step chemistry, identification and characterisation techniques, and thorough understanding of regulatory guidelines to help accelerate drug approval.
Across our global network of cutting-edge facilities, we are able to develop highly effective workflows for our clients and, ultimately, bring life-changing therapeutics to those in need.
Our dedicated process development chemists can help you optimize your drug development and manufacturing processes.
We can deliver quality analytical methods from early phase clinical research to GMP scale-up and commercialization, in line with ICH and FDA/MHRA requirements.
Leveraging our world-leading capabilities in polymorphs, salt forms, crystal morphology and controlled particles, we can help you advance your solid form science.
Building on our long history of navigating the pharmaceutical pipeline, we ensure that all processes comply with global regulatory standards.
From handling procedures to large-scale manufacture, we have extensive experience in developing highly potent APIs.
Our teams can help you deliver and scale up processes for linking small molecule payloads to polymers in support of antibody-drug conjugates (ADCs), polymer-drug conjugates and other drug delivery applications.
Throughout our global facilities and GMP-compliant bulk production plant network, we have the expertise to secure a reliable supply of controlled substance APIs.
We were honored to welcome Nobel Prize laureate, Professor of Chemistry at Stanford University, and Veranova Advisory Board member, Carolyn Bertozzi to our SCALE 2025 Symposium event in July.
Listen to Carolyn’s talk where she discusses her decades-long work developing bioorthogonal and click chemistries, and how these innovations are enabling the construction of complex therapeutic modalities across pharma today.
Read our latest white paper highlighting critical analytical techniques necessary for the characterization of ADCs, emphasizing their physicochemical properties, biological activity, stability assessments, and regulatory implications.
In recent years, interest in psychedelics for therapeutic use has increased. Among these is psilocybin, the world’s most studied psychedelic compound for the treatment of mental health conditions. This Expert Insight explores the potential of this molecule, and the challenges associated with synthesizing it at scale.
Discover new ways to advance your science with Veranova.
Contact us