Designer PEG and Lipid Drug Moieties
Drug design may leverage pegylation or lipid-like functionalization for controlling pharmacokinetics and bioavailability and/or acting as a drug linker group (Figure 1). The hydrophilicity of drug compounds affects the uptake and efficacy of therapy and may be tailored by incorporating PEGs or lipids in pharmaceuticals. These moieties may impart lipophilicity, hydrophilicity, or amphiphilicity as well as modify molecular flexibility and/or provide additional functionalization points. Therefore, the molecular inclusion of PEGs and lipids in drugs serves as a powerful tool for controlling solubility, cell permeability, and mechanistic effects.
PEG and lipid molecules are readily tailored to specific functions and targeted delivery. The molecular length and composition are easily tuned and synthetically accessible, which enables rational design for best performance.
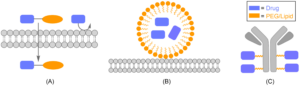
Figure 1: Drug Delivery Systems Containing PEGs or Lipids. (A) PEG/Lipid Prodrug (B) Lipid Based Nanocarrier (C) PEG/Lipid Linker for Antibody Drug Conjugate (ADC)
Custom PEG and Lipids Synthesis
Veranova has over 20 years of experience in custom synthesis of PEG and lipid drug linkers and modifiers. The demonstrated products span development to commercial phase for antibody drug conjugates, oligonucleotides, peptides, highly potent APIs, and other small molecule APIs for control of solubility, physical properties, and pharmacokinetics.
Crystallization of Flexible Drug Moieties
Flexible molecules containing PEG moieties may be crystallized by forming coordination complexes (i.e., ionic cocrystals) to improve stability and purity. Commonly, these molecules are isolated as amorphous gums or liquids, whereas crystallization results in a solid that can be isolated by filtration. This not only generates cost savings, improved efficiency, and increased stability but can also eliminate the need for further purification. Veranova has developed a unique screening workflow for crystallization conditions and techniques as well as solid form characterization to enable this technology.
We have leveraged our industry-leading expertise in solid state science and particle engineering to develop methods for ionic cocrystal formation of various size PEGs as shown in Figure 2. The crystal structures were determined by X-ray powder diffraction (XRPD) in-house and established that the ethylene glycol units are coordinating metal ions with water.
Veranova’s approaches and unique capabilities can be extended to other flexible molecules which are difficult to crystallize like lipids and peptides.
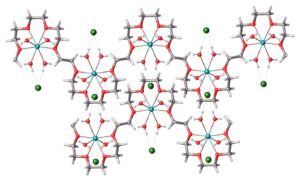
Figure 2: Crystal Packaging of Ionic Cocrystal PEG (PEG, metal ion, and water with counter ion)
Additional Chromatography and Purification Development
Additional separations and isolation techniques are developable with Veranova’s vast experience spanning small to large molecules (e.g., ADC intermediates, flexible linkers, polymers). Our separations team together with the analytical, chemical development, and solid form experts can determine conditions for rapid purification and impurities identification at all scales. We screen a broad scope of techniques to determine the ideal method. These methods are researched at lab scale, modelled for scale up, then verified and implemented at large scale. For example, we routinely accomplish low molecular weight impurity removal by tangential flow filtration (TFF) and impurity isolation, characterization, and removal by high-pressure liquid chromatography (HPLC) and solid phase extraction (SPE). Each method is scalable to commercial GMP production.